
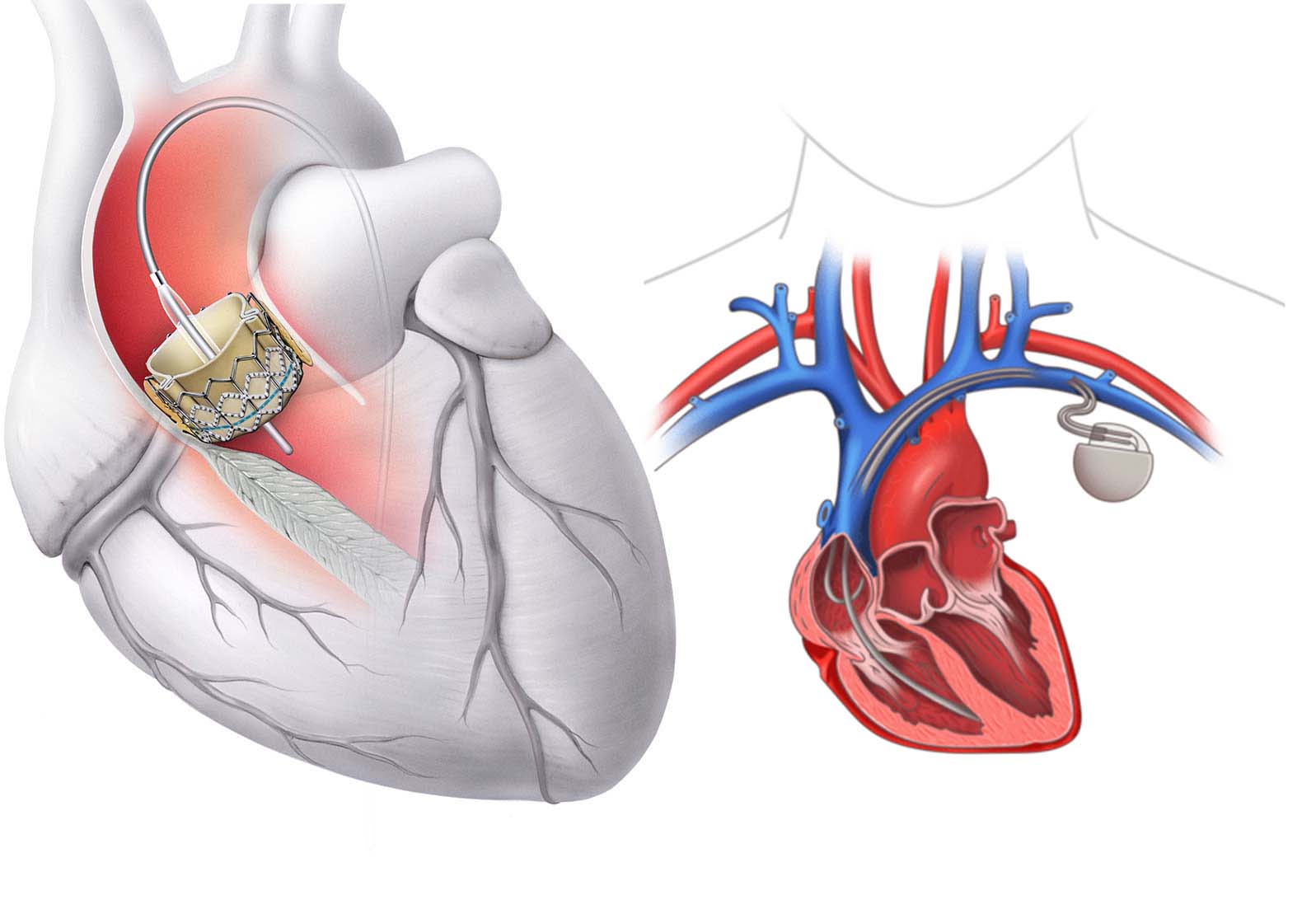
As transcatheter aortic valve implantation (TAVI) becomes the gold standard for treating aortic stenosis across a growing range of patient risk categories, new challenges are emerging—chief among them, conduction disturbances.
Despite procedural success, atrioventricular (AV) block, new-onset left bundle branch block (LBBB), and high-degree heart block remain common complications after TAVI. These electrical disruptions can impact long-term patient outcomes, mandate permanent pacemaker implantation (PPI), and even affect mortality.
In this blog, we unpack the why, who, and when of post-TAVI conduction issues—and how a thoughtful approach to monitoring and pacing can improve care.
Why Do Conduction Disturbances Occur After TAVI?
The aortic valve annulus lies in close proximity to the His-Purkinje conduction system, particularly along the membranous septum. Mechanical stress, radial force, and local trauma during valve implantation can easily disrupt electrical conduction, especially with:
Incidence and Clinical Relevance
While many disturbances resolve spontaneously, some persist—and inappropriately timed pacemaker decisions may lead to unnecessary device implantation or sudden deterioration.
Key Risk Factors for Post-TAVI Conduction Disturbance
|
Patient Factors |
Anatomical Factors |
Procedural Factors |
|
Pre-existing RBBB or bifascicular block |
Short membranous septum |
Deep prosthesis implantation |
|
Baseline PR or QRS prolongation |
Heavy LVOT or annular calcification |
Valve oversizing |
|
Older age and frailty |
Small aortic annulus |
Use of self-expanding valve systems |
Clinical Tip: Patients with RBBB and baseline prolonged PR interval are at the highest risk for complete heart block post-TAVI.
Post-TAVI Monitoring Strategy
A structured monitoring plan is essential to guide appropriate pacing decisions.
In-Hospital Monitoring (Day 0–3)
If LBBB develops, monitor for QRS widening and symptoms of AV block.
🔬 High-Risk Indicators During Monitoring:
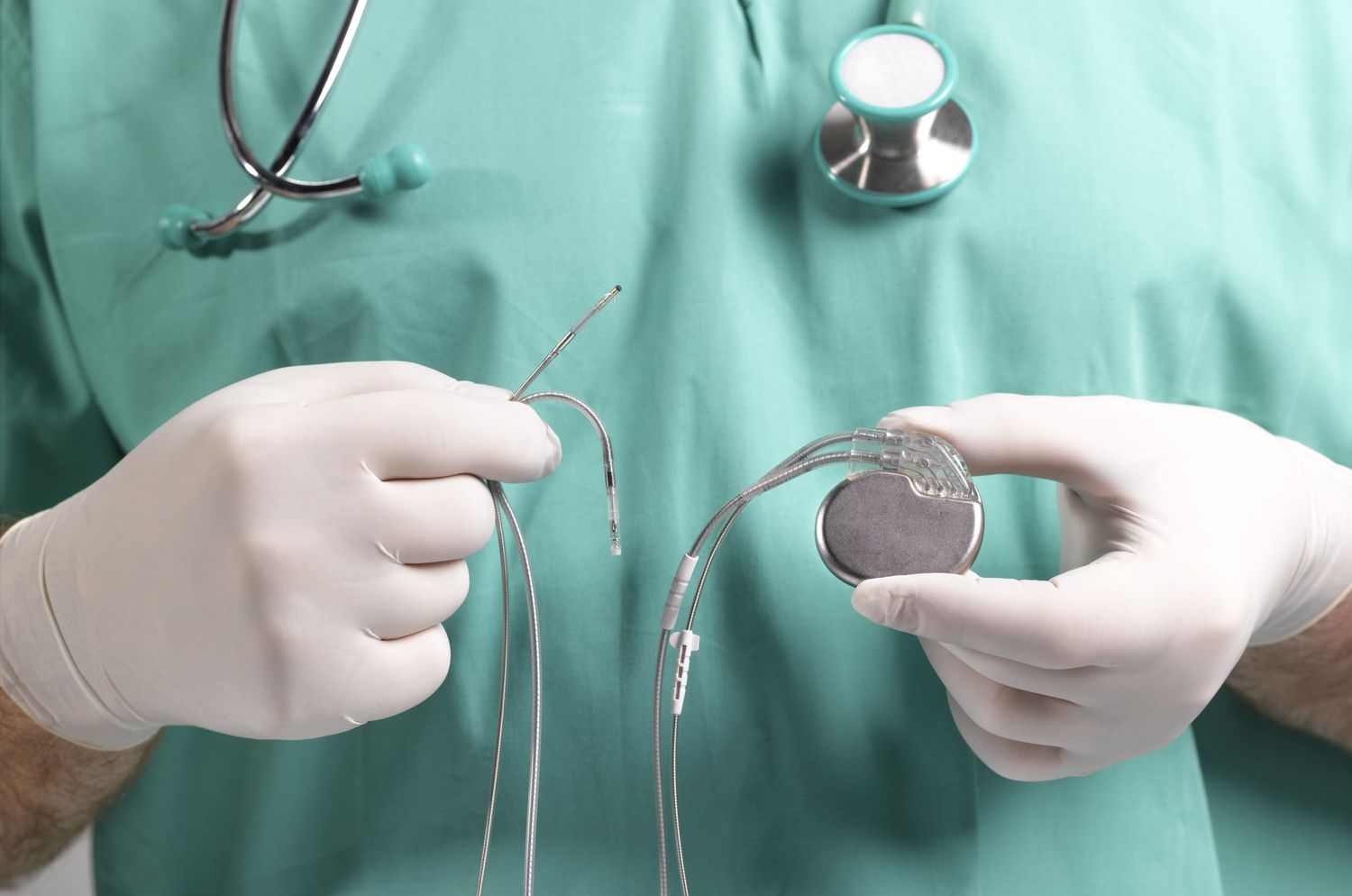
When Should You Implant a Permanent Pacemaker?
Clear Indications (Implant Immediately):
Watchful Waiting (With Close Monitoring):
Suggested Observation Period: 48–72 hours post-TAVI for borderline cases; consider ambulatory Holter or ILR if early discharge is planned.
Valve Type and Pacing Risk
|
Valve Type |
Pacing Risk Profile |
|
Self-expanding (e.g., CoreValve) |
Higher risk (~15–25% PPI rate) |
|
Balloon-expandable (e.g., Sapien) |
Lower risk (~5–10% PPI rate) |
|
Mechanically-expandable (e.g., Lotus) |
High risk but withdrawn from market |
Implantation depth matters more than valve type alone. Deep placement (≥6 mm below annulus) significantly increases conduction risk.
Long-Term Implications of Post-TAVI Pacing
While pacemakers prevent bradyarrhythmic complications, they are not benign. Chronic RV pacing has been associated with:
This underscores the need to avoid unnecessary implantation and consider alternatives such as His-bundle or biventricular pacing in select patients.
Final Thought: Balance Speed with Strategy
TAVI teams must walk a fine line between reacting to early conduction changes and premature pacemaker implantation.
The key is structured monitoring, individualized risk assessment, and clear pacing algorithms.
At TriVasc Academy, we emphasize a team-based, evidence-informed approach to conduction management—because saving a heart valve means little if we destabilize the heart’s rhythm in the process.
Coming Soon at TriVasc Academy:
Post-TAVI conduction monitoring checklists
Pacemaker implantation decision trees
Annotated ECGs and real-world case series
The content provided in this article and throughout the Trivasc Academy platform is intended for educational and informational purposes only. It does not constitute medical advice, diagnosis, or treatment, nor is it intended to replace the clinical judgment of qualified healthcare professionals. All clinical decisions—especially those concerning patient care, procedural planning, or surgical interventions—must be made by board-certified and appropriately credentialed medical practitioners based on their own professional expertise, institutional protocols, and applicable regional regulations.
While every effort has been made to ensure the accuracy, currency, and relevance of the information presented, Trivasc Academy makes no representations or warranties, express or implied, regarding the completeness, applicability, or clinical appropriateness of the content. We assume no responsibility or liability for any direct, indirect, incidental, or consequential harm, loss, or damage resulting from the use of any information or guidance provided herein.
Trivasc Academy does not endorse any specific device, technology, or clinical approach mentioned unless explicitly stated, and any reference to commercial products or services is for educational illustration only. Readers are strongly encouraged to consult official guidelines, product IFUs, and institutional policies before implementing any technique or procedure discussed on this site.
Use of this website and its content constitutes agreement to these terms. For full legal terms, please refer to our Terms of Use and Privacy Policy.
© 2025 TriVasc Academy. All content Copyrighted. All trademarks reserved.